Bacterial filamentation: a bet for survival in stressful environments
About me
Genomic Scientist / Data Scientist
Self-taught learner.
Lover of reproducibility.
Software development enthusiast.

My goal is to create tools that help us to expand the knowledge
Thanks to my colleagues!




Before we begin, let’s review a few key concepts
What is mathematical modelling?

What are antibiotics?

What is the SOS system?

So… What does all this have to do with bacterial filamentation?

Problem

Why did the bacteria elongate?

Mathematical modelling approximation
Mathematical modelling approximation

Cell dimensions relationship

Cell dimensions relationship
A cell can be represented as a capsule
\[ Surface\:area = 2 \cdot \pi \cdot radius\;(2 \cdot radius + side\:length) \\ Volume = \pi \cdot r^2\:(4/3 \cdot radius + side\:length)\\ \]
Cell dimensions relationship
- Cells tend to increase their side length, but not their radius.
Cell dimensions relationship

What would happen if we consider the cell growth of a bacterium in the face of exposure to a toxic agent?
.
Filamentation model
\[ \begin{split} \frac{dT_{int}}{dt} &= T_{sa} \cdot (T_{ext}(t) - T_{vol}) - \alpha \cdot T_{ant} \cdot T_{int} \\ \frac{dL}{dt} &= \begin{cases} \beta \cdot L,& \text{if } T_{int} \geq T_{sos}, t \geq \tau_{sos} + \tau_{delay} \text{ and } L < L_{max} \\ 0, & \text{otherwise} \end{cases} \end{split} \]
where
\(T_{int}\) = Internal toxin
\(L\) = Cell length
Numerical results
Filamentation provides transient resistance to stressful conditions
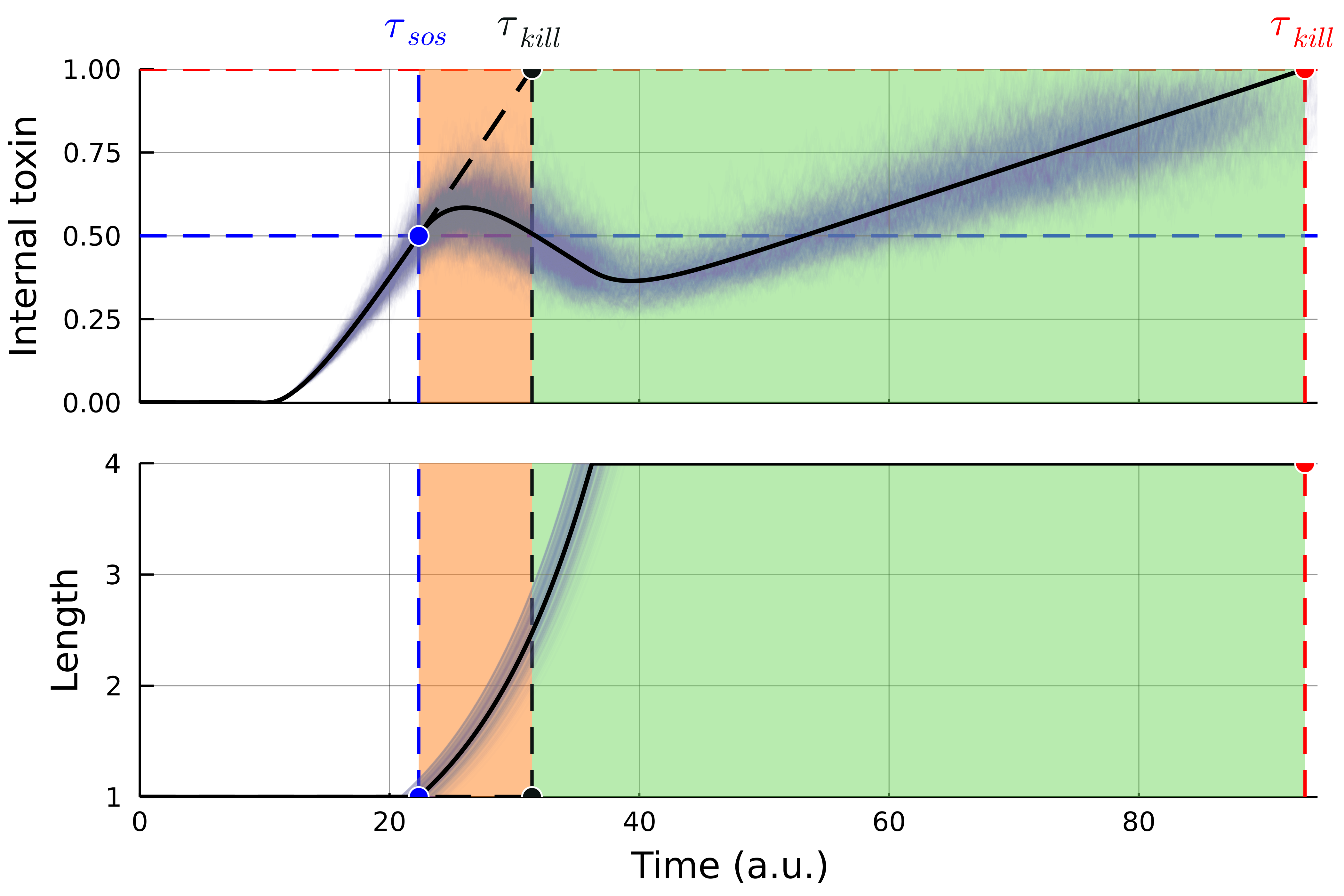
Filamentation increases the minimum inhibitory concentration
Heterogeneity in the toxin-antitoxin system represents a double-edged sword in survival probability
Heterogeneity in the toxin-antitoxin system represents a double-edged sword in survival probability
Experimental results
Single-cell analysis of a semi-lethal pulse

Single-cell analysis of a semi-lethal pulse
Experimental model: E. coli K12 MG1655 bearing a \(ColE1-like\) (p15a) plasmid, pBGT, encoding for the \(β-lactamase\) resistance gene \(bla_{TEM-1}\) that confers resistance to ampicillin, an \(eGFPmut2\) gene under an arabinose inducible promoter, and the \(araC\) repressor.
Control model: E. coli K12 MG1655 was used, carrying the \(pBADgfp2\), \(araC\), and the \(bla_{TEM-1}\) integrated into the chromosome through the \(λ-phage\).
Single-cell analysis of a semi-lethal pulse

Single-cell analysis of a semi-lethal pulse - Image processing

Summary of results obtained after tracking individual cells lineages
Cell length and the amount of GFP are crucial for determining cell survival
Cell length and the amount of GFP are crucial for determining cell survival
Time to reach filamentation matters for determining cell survival
PCA emphasizes the importance of cell length and its GFP in cell survival
PCA emphasizes the importance of cell length and its GFP in cell survival
UMAP correctly represents the local structure of cell status
Population dynamics reveal how filamentation contributes to cell survival
Population dynamics reveal how filamentation contributes to cell survival
Population dynamics reveal how filamentation contributes to cell survival

Heterogeneity in plasmid copy-number allows various forms of survival in addition to filamentation

Heterogeneity in plasmid copy-number allows various forms of survival in addition to filamentation
Carrying plasmids is associated with a fitness cost in non-selective conditions

Recapitulation of experimental analysis

Science must be open and reproducible to anyone who wants to learn more
Science must be open and reproducible to anyone who wants to learn more
Thanks to my colleagues!




Questions?

References

@jvelezmagic